
Chemistry, 12.04.2021 21:00 zappygal923
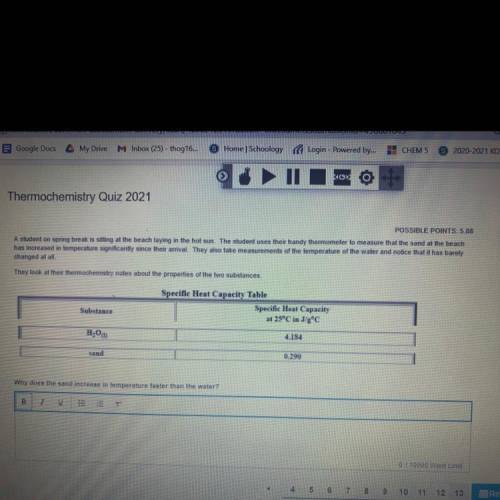
A student on spring break is sitting at the beach laying in the hot sun. The student uses their handy thermometer
to measure that the sand at the beach has increased in temperature significantly since their arrival. They
also take measurements of the temperature of the water and notice that it has barely changed at all.
They look at their thermochemistry notes about the properties of the two substances,
Why does the sand increase in temperature faster than the water?
PLZZZ HELPPP!! ASAP

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
A student on spring break is sitting at the beach laying in the hot sun. The student uses their hand...
Questions

Mathematics, 29.04.2021 19:40






Mathematics, 29.04.2021 19:40


Mathematics, 29.04.2021 19:40



Mathematics, 29.04.2021 19:40





Mathematics, 29.04.2021 19:40

History, 29.04.2021 19:40




