Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

You know the right answer?
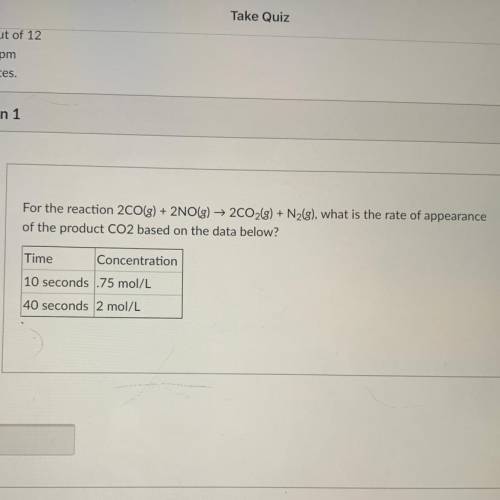
For the reaction 2CO(g) + 2NO(g) → 2C02(g) + Nzg), what is the rate of appearance
of the product CO...
Questions

Mathematics, 13.05.2021 16:40


Mathematics, 13.05.2021 16:40

English, 13.05.2021 16:40


Mathematics, 13.05.2021 16:40



English, 13.05.2021 16:40



Chemistry, 13.05.2021 16:40

Social Studies, 13.05.2021 16:40


Social Studies, 13.05.2021 16:40



History, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40

Mathematics, 13.05.2021 16:40