
Chemistry, 12.04.2021 21:50 twiddleturd
In this experiment, you will have a chance to test the hypothesis that Ernest Rutherford used when determining the size of the nucleus. In his "gold foil experiment," Rutherford shot alpha particles at gold atoms. Once he realized that the alpha particles were hitting a concentrated positive mass, he developed the nuclear model of the atom. Next, he set out to determine the relative size of the nucleus compared to the rest of the atom. He reasoned that the smaller the nucleus, the less likely it was to be hit by an alpha particle. This led to a simple comparative ratio:
It took a great number of shots to actually hit the nucleus because the size of the atom was so much larger than the nucleus. Rutherford proposed that the "hit ratio" was approximately equal to the volume ratio. This is the hypothesis you will test in this experiment.
OBJECTIVES
Investigate a scientific hypothesis.
Present your findings in a scientific report.
Online Lab
The animation will help you test Rutherford’s hypothesis. Be sure to record the dimensions of the box and the block so that you can find their volumes when you present your findings.
00:4001:33
SHOW TRANSCRIPT
Present Your Findings
When you are finished with the experiment, complete the following data analysis and record your answers in the Essay box below.
Determine the volume of the box and the block.
Determine the ratio of the block to the box:
Multiply this number by 100 to turn it into a percent.
Complete this statement: The volume of the block is _ percent of the volume of the box.
Determine the ratio of the number of hits to the number of shots:
Multiply this number by 100 to turn it into a percent.
Complete this statement: The block was hit _ percent of the time.
Compare the results of step 2 to the results of step 3. Are the percentages similar?
Write a conclusion discussing the following items:
Based on your findings, do you think Rutherford's hypothesis was reasonable?
Restate Rutherford's hypothesis and describe how you tested it.
State whether your results support the hypothesis. If they do not, can you suggest some error in experimental procedure (other than general human error) that might explain it?
Finally, explain how this experiment confirms the nuclear model of the atom and the idea that most of the atom is empty space.
Length of box 20.75 in.
Width of box 14.25 in.
Height of box 12 in.
Length of block 2.5 in.
Width of block 1 in.
Height of block 1 in.
Number of hits on the block 2
Total number of shots 100
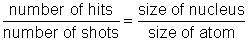
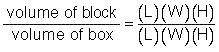


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3


Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
In this experiment, you will have a chance to test the hypothesis that Ernest Rutherford used when d...
Questions

Mathematics, 01.01.2022 03:20




Social Studies, 01.01.2022 03:20


Mathematics, 01.01.2022 03:20


Chemistry, 01.01.2022 03:20

History, 01.01.2022 03:20

Arts, 01.01.2022 03:20












