
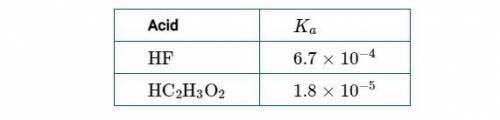
Chemistry, 12.04.2021 22:00 macylen3900
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K for the process represented by the equation H2O(l)⇄H2O(g).
(b) Considering your answer to part (a), indicate whether the process is thermodynamically favorable at 298K. Justify your answer.
(c) Considering your answer to part (b), explain why H2O(l) has a measurable equilibrium vapor pressure at 298K.
(2nd Screenshot)
Water vapor can be produced in two different processes, as represented below.
(d) In terms of concepts of entropy and the particle-level structure of the different phases of water, explain why the change in entropy, ΔS, is greater for process 1 than for process 2.
Please help as soon as possible


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

You know the right answer?
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K f...
Questions

Mathematics, 26.07.2019 12:10

Advanced Placement (AP), 26.07.2019 12:10

Mathematics, 26.07.2019 12:10


Health, 26.07.2019 12:10








Social Studies, 26.07.2019 12:10

Chemistry, 26.07.2019 12:10


Computers and Technology, 26.07.2019 12:10

History, 26.07.2019 12:10


Mathematics, 26.07.2019 12:10

Mathematics, 26.07.2019 12:10



