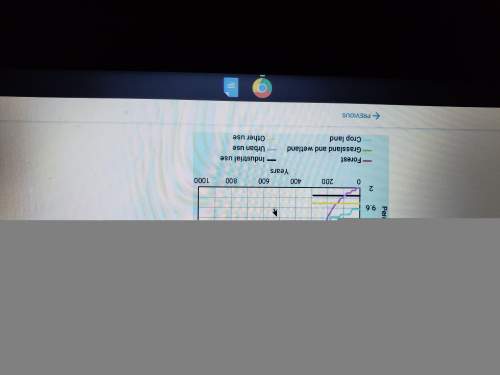
Which of the following statements is/arel correct:
A. Entropy increases with the number of energetically equivalent ways to arrange the components of a system.
B. A given set of conditions such as P, V, T defines a microstate of a system.
C. The change represented below has a positive AS. 04
a. A only
b. A and C only
c. A and B only
d. A, B, and C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1


Chemistry, 23.06.2019 07:40
Did the detergents containing enzymes work better at removing stains than those containing no enzyme? why or why not?
Answers: 2

You know the right answer?
Which of the following statements is/arel correct:
A. Entropy increases with the number of energeti...
Questions

Computers and Technology, 07.10.2019 21:00


Health, 07.10.2019 21:00


English, 07.10.2019 21:00


English, 07.10.2019 21:00


Mathematics, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00

Arts, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

German, 07.10.2019 21:00

Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00



Mathematics, 07.10.2019 21:00

English, 07.10.2019 21:00