
Chemistry, 13.04.2021 02:10 Misspaige4453
It is time to practice using potential energy diagrams. Respond to the three questions below on energy diagrams and submit to your instructor.
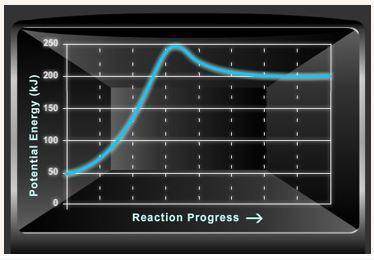
Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time.
Does this graph represent an endothermic or an exothermic reaction? Explain your answer.
What is the enthalpy change, ΔH, for this reaction? Show your work.
What is the activation energy, Ea, for this reaction? Show your work.
In a particular chemical reaction, the energy of the reactants is 30 kJ and the energy of the products is 5 kJ. The maximum energy of the system is 40 kJ.
Sketch a potential energy diagram for this reaction. Make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpy change for the reaction.
What is the activation energy for this reaction?
What is the enthalpy change for this reaction?
Is this reaction endothermic or exothermic? Explain your answer in two ways: first, using the energy values, and second, by referring to the shape of the graph.
The coating on the head of a match is highly flammable. When it burns, it releases a great deal of energy. However, before the match can burn, it must gain a small amount of energy from a spark. That spark is typically produced by striking (rubbing) the match head against a rough surface. Sketch and describe a potential energy diagram that represents the striking and burning of the match. Remember to label the diagram with the energy changes that occur. Your answer must include the potential energy diagram and a written description. (Note: you do not have to use actual energy values.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 21.06.2019 22:50
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas.in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
It is time to practice using potential energy diagrams. Respond to the three questions below on ener...
Questions




History, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01



Computers and Technology, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

Computers and Technology, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

Social Studies, 16.10.2020 07:01

History, 16.10.2020 07:01





