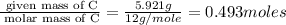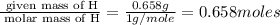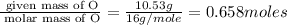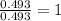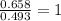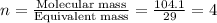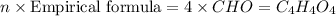A 17.11 gram sample of an organic compound containing only C, H, and O is analyzed by combustion analysis and 21.71 g CO2 and 5.926 g H2O are produced. In a separate experiment, the molar mass is found to be 104.1 g/mol. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C, H, O empirical formula

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science)
Answers: 3
You know the right answer?
A 17.11 gram sample of an organic compound containing only C, H, and O is analyzed by combustion ana...
Questions

History, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01




Chemistry, 22.10.2020 19:01



Mathematics, 22.10.2020 19:01


Mathematics, 22.10.2020 19:01




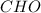 and
and 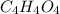 respectively.
respectively.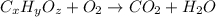
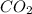 = 21.71 g
= 21.71 g
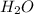 = 5.926 g
= 5.926 g
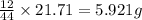 of carbon will be contained.
of carbon will be contained.
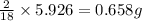 of hydrogen will be contained.
of hydrogen will be contained.