Chemistry, 13.04.2021 02:30 evelyngarcia99
Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . Suppose 1.5 g of hydrochloric acid is mixed with 2.67 g of sodium hydroxide. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2
You know the right answer?
Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride...
Questions


Biology, 02.07.2019 17:10







English, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10



Mathematics, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10

History, 02.07.2019 17:10

Mathematics, 02.07.2019 17:10




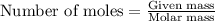 .....(1)
.....(1) 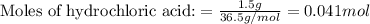
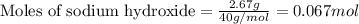

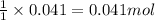 of NaOH
of NaOH
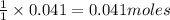 of water
of water