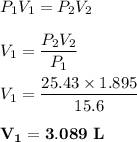A gas that exerts a pressure of
15.6 psi in a container with a volume of
L will exert a press...

Chemistry, 13.04.2021 07:00 tvrgrvJfbhh3881
A gas that exerts a pressure of
15.6 psi in a container with a volume of
L will exert a pressure of
25.43 psi when transferred to a
container with a volume of 1.895 L.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Questions



English, 01.09.2020 17:01

World Languages, 01.09.2020 17:01

Mathematics, 01.09.2020 17:01

Mathematics, 01.09.2020 17:01





History, 01.09.2020 17:01



History, 01.09.2020 17:01

Mathematics, 01.09.2020 17:01

Mathematics, 01.09.2020 17:01




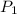 = 15.6 psi
= 15.6 psi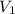 = ???
= ???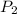 = 25.43 psi
= 25.43 psi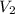 = 1.895 L
= 1.895 L