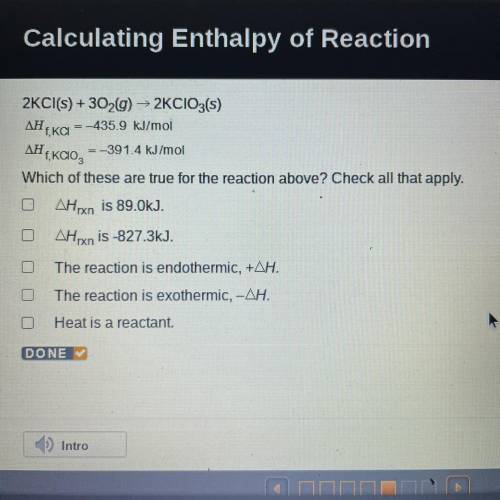
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,<...

Chemistry, 13.04.2021 07:10 Harini5721
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,
Which of these are true for the reaction above? Check all that apply.
AHxn is 89.0kJ.
OAHrxn is -827.3kJ.
The reaction is endothermic, +AH.
O The reaction is exothermic, -AH.
Heat is a reactant.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
Questions



History, 14.11.2019 22:31

History, 14.11.2019 22:31








Computers and Technology, 14.11.2019 22:31





Geography, 14.11.2019 22:31





