
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of
sodium azide, NaN3.
2 NaNz (s) 2 Na (s) + 3 N2 (g)
If an air bag has a volume of 53.4 L and is to be filled with nitrogen gas at a pressure of 1.07 atm and a
temperature of 23.7°C, how many moles of NaNz must decompose? You may assume the Ny behaves as
an ideal gas.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Type the correct answer in the box. spell all words correctly .what does biodiesel produce in higher amounts? biodiesel produces higher amounts
Answers: 2

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1


You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions

Social Studies, 16.11.2020 20:40

History, 16.11.2020 20:40

Social Studies, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40



Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Biology, 16.11.2020 20:40


English, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40

Mathematics, 16.11.2020 20:40


English, 16.11.2020 20:40



English, 16.11.2020 20:40


Biology, 16.11.2020 20:40

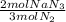 = 1.57 mol NaN₃
= 1.57 mol NaN₃

