
Chemistry, 13.04.2021 18:20 Trucofer8159
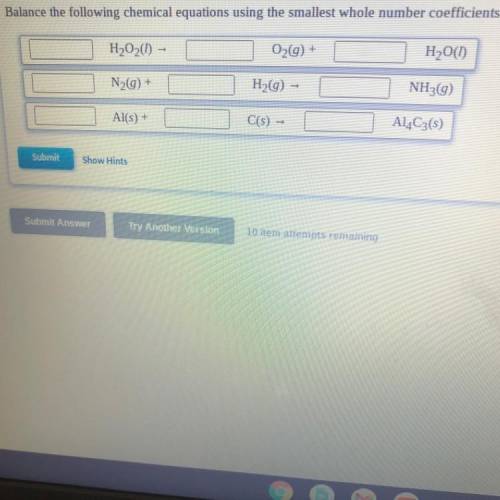
Balance the following chemical equations using the smallest whole number coefficients.
H2O2(I) -
O2(g) +
H2O(1)
N29) +
H2(g) –
NH3(g)
Al(s) +
C(s) -
Al4C3(5)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Balance the following chemical equations using the smallest whole number coefficients.
H2O2(I) -
Questions


Spanish, 17.02.2021 14:10

Physics, 17.02.2021 14:10

Advanced Placement (AP), 17.02.2021 14:10

Biology, 17.02.2021 14:10



History, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

Spanish, 17.02.2021 14:10


Mathematics, 17.02.2021 14:10


Mathematics, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10




English, 17.02.2021 14:10

Law, 17.02.2021 14:10



