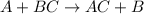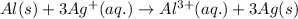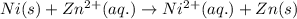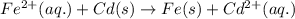Chemistry, 27.09.2019 01:40 ryleerose255
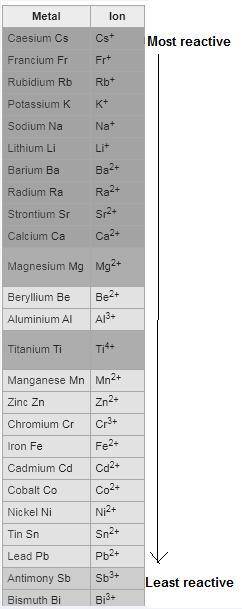
Which of the following redox reactions do you expect to occur spontaneously in the forward direction?
check all that apply.
ni(s)+pb2+(aq)→ni2+(aq)+pb(s)
al(s)+3ag+(aq)→al3+(aq)+3ag(s)
ni(s)+zn2+(aq)→ni2+(aq)+zn(s)
fe2+(aq)+cd(s)→fe(s)+cd2+(aq)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Which of the following redox reactions do you expect to occur spontaneously in the forward direction...
Questions









Spanish, 17.05.2021 01:00


Mathematics, 17.05.2021 01:00




English, 17.05.2021 01:00


Biology, 17.05.2021 01:00


Mathematics, 17.05.2021 01:00

Physics, 17.05.2021 01:00