
CUMULATIVE EXAM!
The graph below compares the total amounts of greenhouse gas emissions expected for future mid-size cars, including emissions that occur when moving fuels from their original source to fueling stations where people can buy the fuels for their cars. Amounts are shown in grams of CO₂ (or equivalent greenhouse gas) per mile driven by the mid-size car.
Which vehicles are the best choices for environmentally friendly cars?
A. fuel cell electric and battery electric
B. hybrid electric and plug-in hybrid electric
C. conventional internal combustion and hybrid electric
D. conventional internal combustion and battery electric
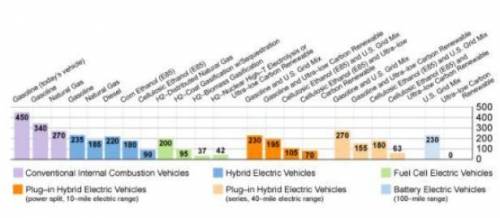

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1
You know the right answer?
CUMULATIVE EXAM!
The graph below compares the total amounts of greenhouse gas emissions expected fo...
Questions



Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20



Chemistry, 30.03.2021 03:20


Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20


Mathematics, 30.03.2021 03:20


Mathematics, 30.03.2021 03:20

Chemistry, 30.03.2021 03:20



Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20



