Help please thank you
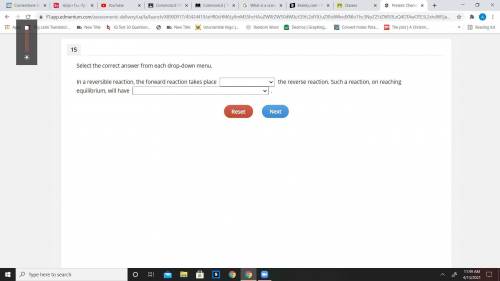
In a reversible reaction, the forward reaction takes place_ the reverse...

Chemistry, 13.04.2021 20:10 taraupchurch23
Help please thank you
In a reversible reaction, the forward reaction takes place_ the reverse reaction. Such a reaction, on reaching equilibrium, will have _.
1st blank
A. after
B. before
c. simultaneously with
2nd blank
A. a constant rate of forward reaction and reverse reaction
B. an equal number of products and reactants
c similar masses of reactants and products

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
Questions

Mathematics, 30.11.2020 06:10

Mathematics, 30.11.2020 06:10

Mathematics, 30.11.2020 06:10

Arts, 30.11.2020 06:10

Biology, 30.11.2020 06:10












Mathematics, 30.11.2020 06:10

History, 30.11.2020 06:10

English, 30.11.2020 06:10

History, 30.11.2020 06:10



