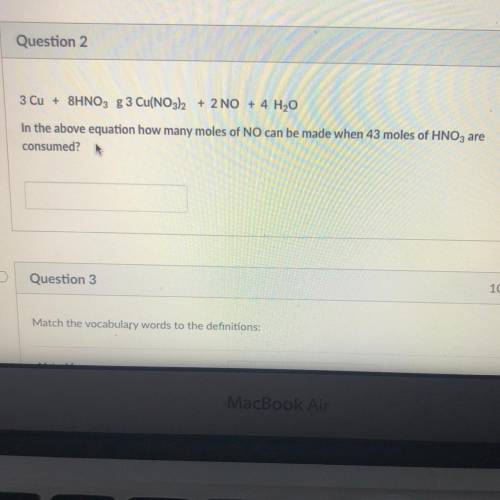
In the above equation how many moles of NO can be made when 43 moles of HNO3 are consumed?
...

Chemistry, 13.04.2021 23:10 arigamez90
In the above equation how many moles of NO can be made when 43 moles of HNO3 are consumed?

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
You know the right answer?
Questions


Chemistry, 30.09.2019 03:20

Chemistry, 30.09.2019 03:20


Physics, 30.09.2019 03:20



Mathematics, 30.09.2019 03:20





Mathematics, 30.09.2019 03:20

Social Studies, 30.09.2019 03:20



Mathematics, 30.09.2019 03:20

Social Studies, 30.09.2019 03:20




