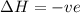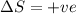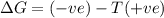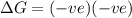Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
What does a process require to be spontaneous at all temperatures? answer a catalyst and lower acti...
Questions

Mathematics, 02.09.2021 01:20

Mathematics, 02.09.2021 01:20



English, 02.09.2021 01:20

History, 02.09.2021 01:20

Mathematics, 02.09.2021 01:20

Mathematics, 02.09.2021 01:20



Mathematics, 02.09.2021 01:20

Advanced Placement (AP), 02.09.2021 01:20

Mathematics, 02.09.2021 01:20


Mathematics, 02.09.2021 01:30

Chemistry, 02.09.2021 01:30

Social Studies, 02.09.2021 01:30


Mathematics, 02.09.2021 01:30

Mathematics, 02.09.2021 01:30

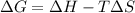
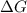 = free energy change
= free energy change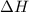 = enthalpy change
= enthalpy change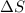 = entropy change
= entropy change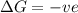 , reaction is spontaneous
, reaction is spontaneous