Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 05:50
Astudent made a graph plotting the progress of a reaction over time. the student forgot to label the y-axis of the graph. a graph is shown with two graph lines. one graph line starts at a higher position on the y axis and slopes downwards towards the right. the other graph line starts at a lower position on the y axis and slopes upwards towards the right. the two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x axis. a vertical line is shown at a point where the two graph lines finally became parallel to the x axis. this vertical line is labeled equilibrium. the title on the x axis is time and an arrow pointing towards the right is shown above time. the title on the y axis is left blank. what best explains the label that the student should use on the y-axis? amount, because as the amount of product decreases, the amount of reactant increases over time. reaction rate, because forward and backward reaction become equal at equilibrium. amount, because the amounts of reactants and products become constant after equilibrium is reached. reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.
Answers: 3


Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
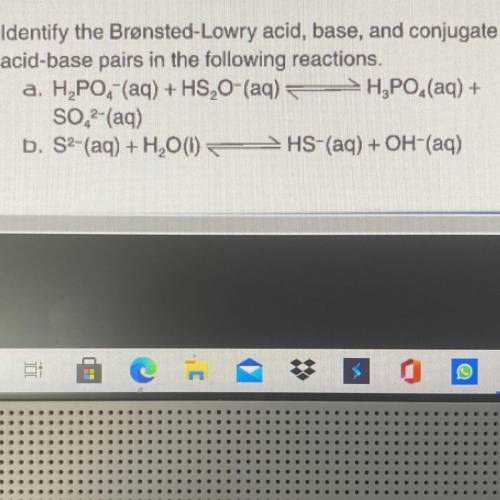
Identify the Bronsted-Lowry Acid, base, and conjugate acid-base pairs in the following reactions. H2...
Questions

Mathematics, 29.10.2020 02:30

English, 29.10.2020 02:30

Mathematics, 29.10.2020 02:30

Chemistry, 29.10.2020 02:30


Mathematics, 29.10.2020 02:30

English, 29.10.2020 02:30



Mathematics, 29.10.2020 02:30

Mathematics, 29.10.2020 02:30



History, 29.10.2020 02:30

Mathematics, 29.10.2020 02:30

History, 29.10.2020 02:30



English, 29.10.2020 02:30