
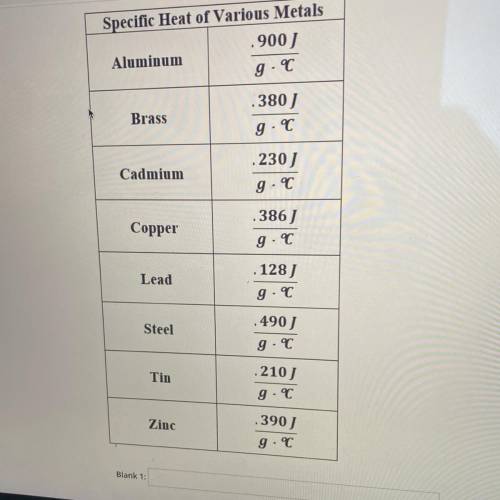
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of water, the water increased temperature from 20.1°C to 35.4°C
If water has a specific heat capacity of 4.18 Jg, determine the unknown metal by calculating it's specific heat. The unknown metal is ___

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of...
Questions



History, 21.07.2019 06:00

History, 21.07.2019 06:00


Physics, 21.07.2019 06:00


History, 21.07.2019 06:00


Biology, 21.07.2019 06:00


English, 21.07.2019 06:00





History, 21.07.2019 06:10





