
Chemistry, 14.04.2021 20:10 Bucsan8688
The equilibrium constant for the reaction shown below is Kc=0.0091. If the equilibrium concentrations of A and B are 0.031 M and 0.0823 M, respectively, what is the equilibrium concentration of C?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2


Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
The equilibrium constant for the reaction shown below is Kc=0.0091. If the equilibrium concentration...
Questions




Mathematics, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50



World Languages, 18.03.2021 01:50


Chemistry, 18.03.2021 01:50

Computers and Technology, 18.03.2021 01:50


Mathematics, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Mathematics, 18.03.2021 01:50

![[C]=0.0221M](/tpl/images/1258/7208/661df.png)
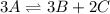
![Kc=\frac{[B]^3[C]^2}{[A]^3}](/tpl/images/1258/7208/b55d9.png)
![[C]^2=\frac{[A]^3Kc}{B]^3}](/tpl/images/1258/7208/13e42.png)
![[C]=\sqrt{\frac{(0.031)^3*0.0091}{(0.0823)^3}}](/tpl/images/1258/7208/c8694.png)



