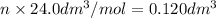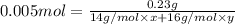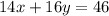Oxides of nitrogen are pollutant gases which are emitted from car exhausts.
in urban tra...

Oxides of nitrogen are pollutant gases which are emitted from car exhausts.
in urban traffic, when a car travels one kilometre, it releases 0.23 g of an oxide of nitrogen nxoy, which occupies 120 cm3.
what are the values of x and y?
(assume 1 mol of gas molecules occupies 24.0 dm3.)
a x = 1, y = 1
b x = 1, y = 2
c x = 2, y = 1
d x = 2, y = 4

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
Questions


Mathematics, 03.05.2021 16:10

Mathematics, 03.05.2021 16:10

Advanced Placement (AP), 03.05.2021 16:10



Chemistry, 03.05.2021 16:10

Spanish, 03.05.2021 16:10


Advanced Placement (AP), 03.05.2021 16:10




Biology, 03.05.2021 16:10

Mathematics, 03.05.2021 16:10


Social Studies, 03.05.2021 16:10


Mathematics, 03.05.2021 16:10

Mathematics, 03.05.2021 16:10

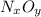 ,m= 0.23 g
,m= 0.23 g
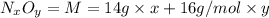
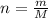
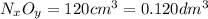
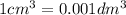
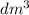 .
.
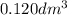 .
.