
Chemistry, 14.04.2021 22:00 cassandraaa
What did I do wrong when I was calculating these values. The answers in the box are my answers but all of them were wrong. First answer gets marked as favorite and 100 points for answering
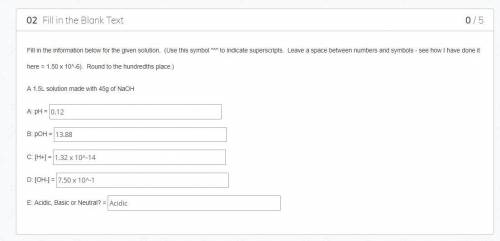

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

You know the right answer?
What did I do wrong when I was calculating these values. The answers in the box are my answers but a...
Questions


Chemistry, 04.12.2020 17:00





Physics, 04.12.2020 17:00





Mathematics, 04.12.2020 17:00

Mathematics, 04.12.2020 17:00

Biology, 04.12.2020 17:00

History, 04.12.2020 17:00







