
Chemistry, 15.04.2021 01:20 raffaldarmaki9412
HURRY
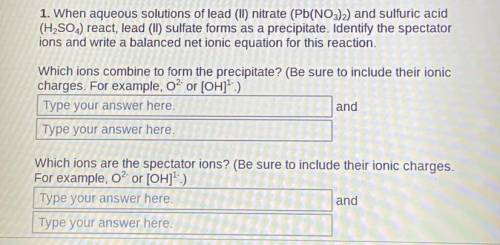
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.
Which ions combine to form the precipitate? (Be sure to include their ionic charges. For example, O² or [OH]¹-)
2. Which ions are the spectator ions? (Be sure to include their ionic charges. For example, O² or [OH]¹-)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
HURRY
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, le...
Questions


Mathematics, 28.01.2020 06:31

Biology, 28.01.2020 06:31


Mathematics, 28.01.2020 06:31




Mathematics, 28.01.2020 06:31

Biology, 28.01.2020 06:31

History, 28.01.2020 06:31



History, 28.01.2020 06:31


Mathematics, 28.01.2020 06:31

Advanced Placement (AP), 28.01.2020 06:31





