Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Please help me to solve this.
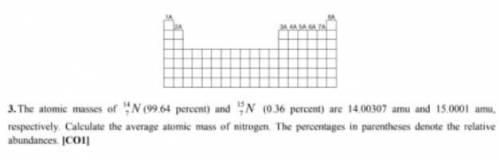
How The atomic musses of 14 N7 (99. 64 percent) and 15N7 (0.36 percen...
Questions

English, 20.09.2020 04:01

Computers and Technology, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01



Business, 20.09.2020 04:01


History, 20.09.2020 04:01



Mathematics, 20.09.2020 04:01

Mathematics, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01