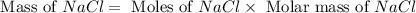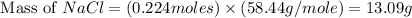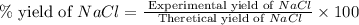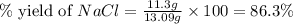Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g
Answers: 2

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
When 15 grams of copper (ii) chloride (cucl2) reacts with 20 grams of sodium nitrate (nano3), 11.3 g...
Questions


Mathematics, 26.03.2021 14:40

Mathematics, 26.03.2021 14:40





Mathematics, 26.03.2021 14:40


Mathematics, 26.03.2021 14:40

Health, 26.03.2021 14:40

English, 26.03.2021 14:40



Mathematics, 26.03.2021 14:40

Social Studies, 26.03.2021 14:40




Business, 26.03.2021 14:40

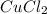 = 15 g
= 15 g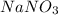 = 20 g
= 20 g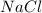 = 58.44 g/mole
= 58.44 g/mole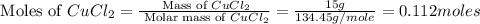
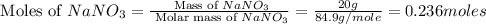
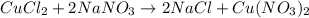
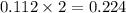 moles of
moles of