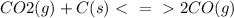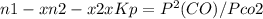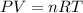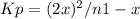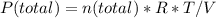Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2


Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 05:40
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
You know the right answer?
Reaction is
co2(g) + c(s) < => 2co(g)
kp = 5.7, 1200k
calcualte the...
co2(g) + c(s) < => 2co(g)
kp = 5.7, 1200k
calcualte the...
Questions

Mathematics, 02.07.2020 01:01



Computers and Technology, 02.07.2020 01:01

Advanced Placement (AP), 02.07.2020 01:01




Mathematics, 02.07.2020 01:01




Mathematics, 02.07.2020 01:01





Computers and Technology, 02.07.2020 01:01