
Chemistry, 15.04.2021 20:00 nkidane2802
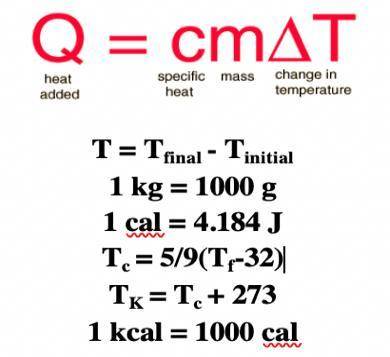
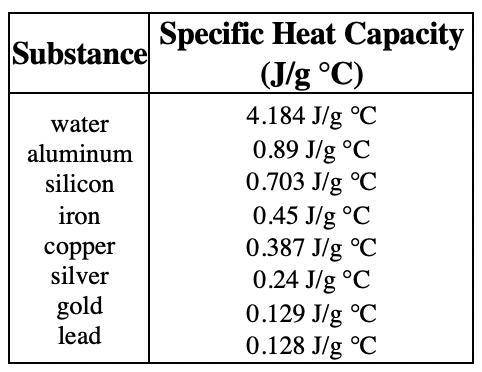
A water heater warms 350 g of water from a temperature of 22.7 oC to a temperature of 83.7 oC. Determine the amount of energy (in joules) required. (Use the table above for missing values)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


You know the right answer?
A water heater warms 350 g of water from a temperature of 22.7 oC to a temperature of 83.7 oC. Deter...
Questions

Biology, 29.03.2021 23:10









Mathematics, 29.03.2021 23:10







History, 29.03.2021 23:10





