
Chemistry, 15.04.2021 20:10 brummy309506
A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one compartment, occupying one-third of the total volume, contains air at 500oR, and the other is evacuated. The valve is opened and the air is allowed to fill the entire volume. Assuming the ideal gas model with variable specific heats. Determine: a. the final temperature of the air (in oR) b. the amount of specific entropy produced (in Btu/lbm oR)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 09:00
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one comp...
Questions

Social Studies, 09.07.2019 14:50

Physics, 09.07.2019 14:50


English, 09.07.2019 14:50



Biology, 09.07.2019 14:50

Mathematics, 09.07.2019 14:50


Mathematics, 09.07.2019 14:50


Mathematics, 09.07.2019 14:50








Mathematics, 09.07.2019 14:50

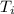
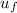 -
- 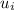 = 0
= 0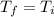
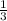 V₂
V₂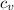 In(
In( 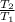 ) + R.In(
) + R.In( 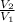 )
) 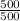 ) + 0.069 × In( 3 )
) + 0.069 × In( 3 )

