Why do weak acid-weak base reactions not go to completion?
there are not enough molecul...

Chemistry, 22.11.2019 04:31 jayycruz59
Why do weak acid-weak base reactions not go to completion?
there are not enough molecules in solution to react with each other.
the conjugate products are much weaker than the original acid and base.
neither a weak acid nor a weak base has a strong tendency to transfer h+ ions.
both weak acids and weak bases have a strong tendency to transfer h+ ions, so they cancel each other out.

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1
You know the right answer?
Questions


Mathematics, 01.11.2019 22:31




Mathematics, 01.11.2019 22:31


History, 01.11.2019 22:31

Mathematics, 01.11.2019 22:31


Mathematics, 01.11.2019 22:31

Mathematics, 01.11.2019 22:31

Mathematics, 01.11.2019 22:31


Mathematics, 01.11.2019 22:31




Mathematics, 01.11.2019 22:31

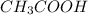 ) is a weak acid and it dissociates poorly in water.
) is a weak acid and it dissociates poorly in water.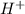 ions.
ions.

