
Chemistry, 15.04.2021 23:20 taylormjensen
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> CO2(g) + CF4(g) K = 2.2 x 10^6
Calculate the equilibrium constant for the following reaction at 25oC:
2CO2(g) + 2CF4(g) <--> 4COF2
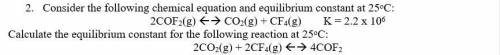

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1
You know the right answer?
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> C...
Questions

Mathematics, 20.12.2023 04:21

English, 20.12.2023 22:57



Mathematics, 05.01.2024 02:27


History, 06.01.2024 23:42












English, 06.03.2024 00:06

Mathematics, 19.03.2024 09:32



