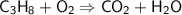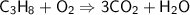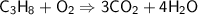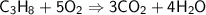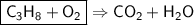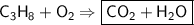This is the equation for the combustion of propane.
c3h8 + 5o2 → 3co2 + 4h2o + heat
which are the reactants and the products in this reaction?
a.
the reactants are c3h8 (propane) and h2o (water). the products are o2 (oxygen) and co2 (carbon dioxide).
b.
the reactants are c3h8 (propane) and o2 ( oxygen). the products are co2 (carbon dioxide) and h2o (water).
c.
the reactants are co2 (carbon dioxide) and h2o (water). the products are c3h8 (propane) and o2 ( oxygen).
d.
the reactants are o2 (oxygen) and co2 (carbon dioxide). the products are c3h8 (propane) and h2o (water).

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

You know the right answer?
This is the equation for the combustion of propane.
c3h8 + 5o2 → 3co2 + 4h2o + heat
whic...
c3h8 + 5o2 → 3co2 + 4h2o + heat
whic...
Questions

History, 20.10.2019 02:30

Mathematics, 20.10.2019 02:30


History, 20.10.2019 02:30


History, 20.10.2019 02:30

English, 20.10.2019 02:30


Mathematics, 20.10.2019 02:30


Geography, 20.10.2019 02:30




Mathematics, 20.10.2019 02:30

Social Studies, 20.10.2019 02:30

Social Studies, 20.10.2019 02:30


History, 20.10.2019 02:30


![\rule[225]{225}{2}](/tpl/images/0178/4900/85973.png)