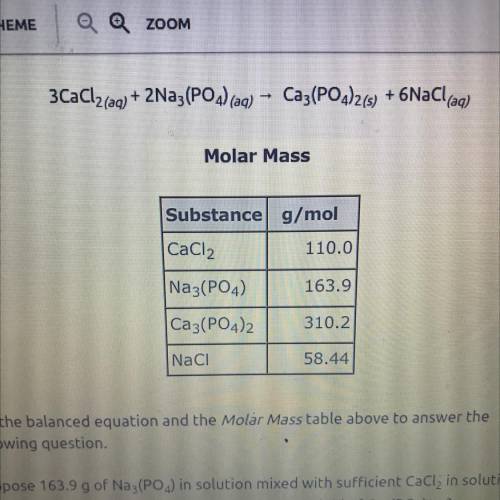
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar...

Chemistry, 16.04.2021 01:00 DragonLovely
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the
following question.
Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl, in solution
yields 116 g of Ca3(PO4)2(s). What is the percent yield of Ca3(PO4)2(5)?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Questions


Mathematics, 05.01.2022 16:20


Mathematics, 05.01.2022 16:20

SAT, 05.01.2022 16:30

Mathematics, 05.01.2022 16:30



Mathematics, 05.01.2022 16:30


Mathematics, 05.01.2022 16:30



English, 05.01.2022 16:30

Mathematics, 05.01.2022 16:40


Mathematics, 05.01.2022 16:40



Mathematics, 05.01.2022 16:40



