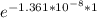Chemistry, 16.04.2021 01:00 BreBreDoeCCx
g Consider (12.5 A) micro-grams of a radioactive isotope with a mass number of (78 B) and a half-life of (32.6 C) million years. If energy released in each decay is 32.6 keV, determine the total energy released in joules (J) in 1 (one) year. Give your answer with three significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1
You know the right answer?
g Consider (12.5 A) micro-grams of a radioactive isotope with a mass number of (78 B) and a half-lif...
Questions




History, 05.05.2020 13:48

English, 05.05.2020 13:48




Mathematics, 05.05.2020 13:48



History, 05.05.2020 13:48

Mathematics, 05.05.2020 13:48


History, 05.05.2020 13:48

History, 05.05.2020 13:48




Mathematics, 05.05.2020 13:48

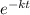
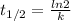
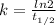
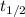 = 32.6 + 18 = 50.6 × 10⁶ years
= 32.6 + 18 = 50.6 × 10⁶ years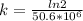 = 1.361 × 10⁻⁸ year⁻¹
= 1.361 × 10⁻⁸ year⁻¹