
Chemistry, 16.04.2021 01:00 davienwatson8
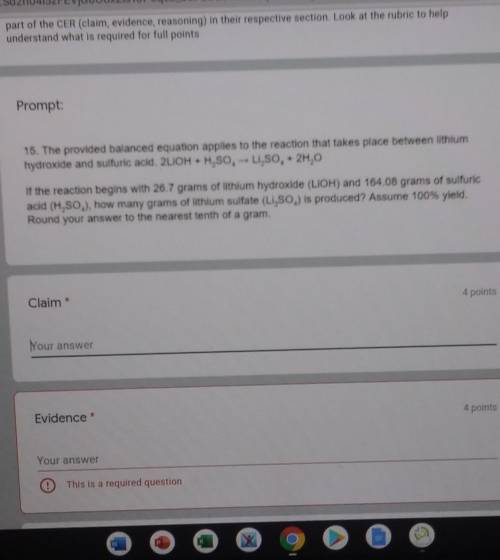
The provided balanced equation applies to the reaction that takes place between lithium, hydroxide and sulfuric acid. 2LiOH + H2SO4 --> Li2SO4 + 2H2O. If the reaction begins with 26.7 grams of lithium hydroxide (LiOH) and 164.048 grams of sulfuric acid (H2SO4), how many grams of lithium sulfate (Li2SO4) is produced? Assume 100% yield. Round your answer to the nearest tenth of a gram

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
The provided balanced equation applies to the reaction that takes place between lithium, hydroxide a...
Questions

English, 01.04.2020 20:44

Mathematics, 01.04.2020 20:44






Mathematics, 01.04.2020 20:44







Spanish, 01.04.2020 20:44

Mathematics, 01.04.2020 20:44

Mathematics, 01.04.2020 20:44

Geography, 01.04.2020 20:44

History, 01.04.2020 20:44




