
Chemistry, 16.04.2021 17:30 SiegeHatake4534
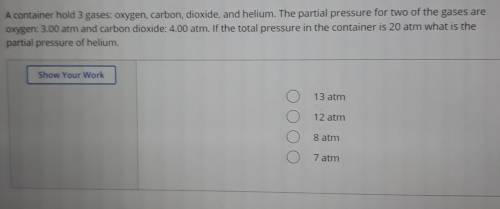
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the gases are oxygen: 3.00 atm and carbon dioxide: 4.00 atm. If the total pressure in the container is 20 atm what is the partial pressure of helium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the g...
Questions


Social Studies, 05.01.2021 22:10

Biology, 05.01.2021 22:10


Mathematics, 05.01.2021 22:10


Mathematics, 05.01.2021 22:10

Social Studies, 05.01.2021 22:10

Biology, 05.01.2021 22:10

Mathematics, 05.01.2021 22:10




Mathematics, 05.01.2021 22:10



Physics, 05.01.2021 22:10

Mathematics, 05.01.2021 22:10


Mathematics, 05.01.2021 22:10



