
Chemistry, 16.04.2021 18:30 Briannadavis03
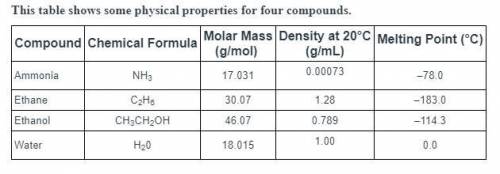
Predict which of these compounds has the highest boiling point.
ammonia, because its low density reduces heat transfer
ammonia, because its low density reduces heat transfer
water, because strong hydrogen bonds form between its molecules
water, because strong hydrogen bonds form between its molecules
ethanol, because its high molecular mass reduces its kinetic energy
ethanol, because its high molecular mass reduces its kinetic energy
ethane, because its low melting point indicates high stability in the liquid phase

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
Predict which of these compounds has the highest boiling point.
ammonia, because its low density re...
Questions


Mathematics, 19.03.2021 22:20

Mathematics, 19.03.2021 22:20

Mathematics, 19.03.2021 22:20

Mathematics, 19.03.2021 22:20

Mathematics, 19.03.2021 22:20


English, 19.03.2021 22:20


Mathematics, 19.03.2021 22:20

Geography, 19.03.2021 22:20

English, 19.03.2021 22:20






Mathematics, 19.03.2021 22:20





