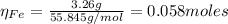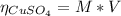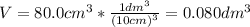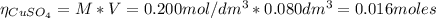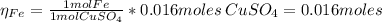Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1
You know the right answer?
2.a. 3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm-3 copper(II)
sulfate solution. The...
Questions

History, 13.07.2019 08:30



Spanish, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30


Biology, 13.07.2019 08:30

History, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

Biology, 13.07.2019 08:30

History, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

History, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

Mathematics, 13.07.2019 08:30

History, 13.07.2019 08:30


History, 13.07.2019 08:30

Chemistry, 13.07.2019 08:30

French, 13.07.2019 08:30

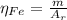
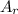 : is the standard atomic weight of iron = 55.845 g/mol
: is the standard atomic weight of iron = 55.845 g/mol