
Chemistry, 16.04.2021 19:10 jaffeisabel
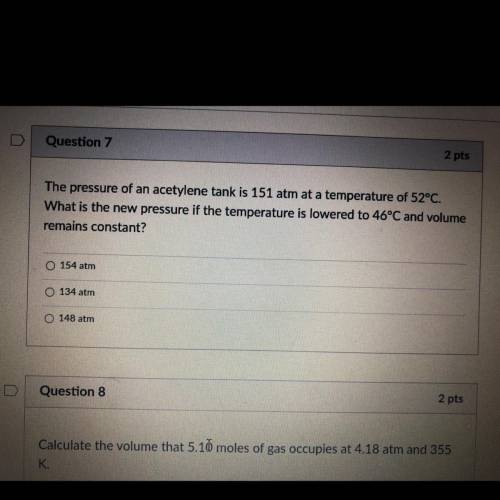
The pressure of an acetylene tank is 151 atm at a temperature of 52°C.
What is the new pressure if the temperature is lowered to 46°C and volume
remains constant?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
The pressure of an acetylene tank is 151 atm at a temperature of 52°C.
What is the new pressure if...
Questions


Mathematics, 09.12.2021 21:30




Mathematics, 09.12.2021 21:30

Computers and Technology, 09.12.2021 21:30

History, 09.12.2021 21:30






World Languages, 09.12.2021 21:30



Mathematics, 09.12.2021 21:30

Mathematics, 09.12.2021 21:30




