
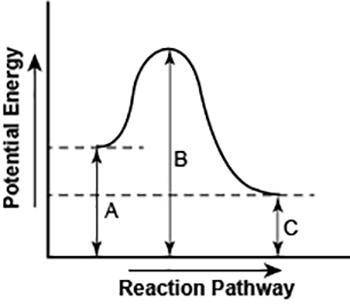
The diagram shows the potential energy changes for a reaction pathway. (10 points)
A curved lined graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The graph curve begins at a higher level and ends at a slightly lower level. A vertical line labeled A, starting from the x axis till the beginning of the graph line is shown. A vertical line labeled B, starting from the x axis till the uppermost hump in the graph curve is shown. Another vertical line labeled C is shown from starting from the x axis till the point where the graph curve ends.
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points)
A curved lined g...
Questions


English, 28.08.2020 20:01

Chemistry, 28.08.2020 20:01









Mathematics, 28.08.2020 20:01



Mathematics, 28.08.2020 20:01


English, 28.08.2020 20:01





