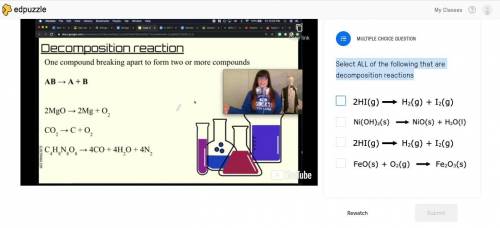
Select ALL of the following that are decomposition reactions
2HI(g)--->H2(g)+I2(g)
Ni(OH...

Chemistry, 16.04.2021 23:00 sairaanwar67
Select ALL of the following that are decomposition reactions
2HI(g)--->H2(g)+I2(g)
Ni(OH)2(s)--->NiO(S)+H2O(I)
2Hi(g)--->H2(g)+i2(g)
FeO(s)+O2(g)--->Fe2O3(s)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2
You know the right answer?
Questions

Arts, 04.12.2020 04:40


Computers and Technology, 04.12.2020 04:40

Mathematics, 04.12.2020 04:40


English, 04.12.2020 04:40

Business, 04.12.2020 04:40

Advanced Placement (AP), 04.12.2020 04:40




Mathematics, 04.12.2020 04:40

Mathematics, 04.12.2020 04:40



Mathematics, 04.12.2020 04:40


Mathematics, 04.12.2020 04:40

English, 04.12.2020 04:40



