Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1

Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2
You know the right answer?
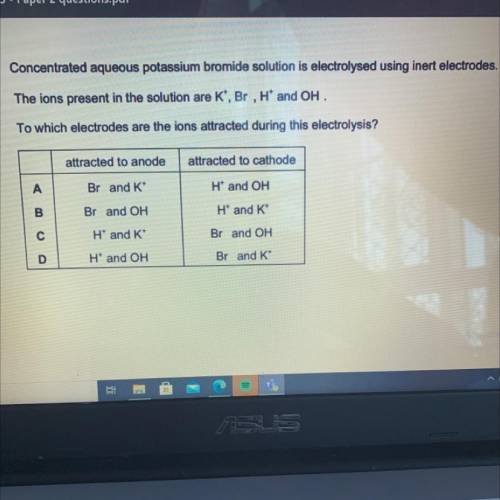
Concentrated aqueous potassium bromide solution is electrolysed using inert electrodes.
The ions pr...
Questions


Advanced Placement (AP), 22.07.2019 08:40

Biology, 22.07.2019 08:40


History, 22.07.2019 08:40




History, 22.07.2019 08:40

Computers and Technology, 22.07.2019 08:40

Mathematics, 22.07.2019 08:40


Mathematics, 22.07.2019 08:40

History, 22.07.2019 08:40

Geography, 22.07.2019 08:40

Geography, 22.07.2019 08:40

Mathematics, 22.07.2019 08:40