
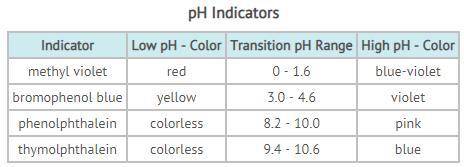
PH is an indicator of how acidic or basic a substance is. The pH scale ranges from 0 - 14, with 7 representing neutral. Acids have a pH below 7; bases, a pH greater than 7. Scientists determine the pH of a substance by using an indicator; indicators change color depending on the pH of a substance. Different indicators work at different pH ranges, as seen in the table above. The transition range of an indicator is the range of pH when the indicator changes from its low-pH color to its high-pH color. If the indicator shows a color between the two, then the pH lies somewhere in that transition range. You have been given a liquid that turns pink in phenolphthalein and blue in thymolphthalein. Tests with methyl violet and bromophenol blue resulted in a violet color. Based on your test results, determine which statements regarding the liquid are accurate. Choose ALL that apply.
A. The liquid is a base.
B. The liquid is an acid.
C. The liquid has a pH > than 7.
D. The liquid is an H⁺ donor.
E. The liquid mostly likely contains OH⁻.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
PH is an indicator of how acidic or basic a substance is. The pH scale ranges from 0 - 14, with 7 re...
Questions

Geography, 17.07.2019 19:30



Mathematics, 17.07.2019 19:30


Computers and Technology, 17.07.2019 19:30


Social Studies, 17.07.2019 19:30




Mathematics, 17.07.2019 19:30

History, 17.07.2019 19:30


Biology, 17.07.2019 19:30


Mathematics, 17.07.2019 19:30


Mathematics, 17.07.2019 19:30



