
Chemistry, 18.04.2021 01:00 raquelle66
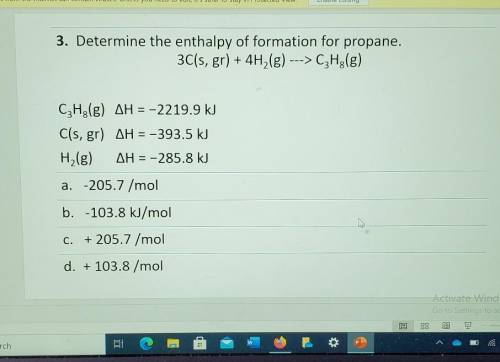
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH = -2219.9 kJ C(s, gr) AH = -393.5 kJ H,(g) AH = -285.8 kJ a. -205.7 /mol b. -103.8 kJ/mol Å C. + 205.7 /mol d. + 103.8 /mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1



Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH =...
Questions


Mathematics, 24.08.2019 21:50


Physics, 24.08.2019 21:50


Chemistry, 24.08.2019 21:50


English, 24.08.2019 21:50




Mathematics, 24.08.2019 21:50

Mathematics, 24.08.2019 21:50



Mathematics, 24.08.2019 21:50

Biology, 24.08.2019 21:50


History, 24.08.2019 21:50

Health, 24.08.2019 21:50



