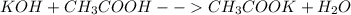Chemistry, 18.04.2021 02:10 firenation18
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titration experiment. Calculate the molarity of the acetic acid solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titra...
Questions

Biology, 13.02.2020 05:51

Mathematics, 13.02.2020 05:51







Social Studies, 13.02.2020 05:51


Computers and Technology, 13.02.2020 05:51

Social Studies, 13.02.2020 05:51



Mathematics, 13.02.2020 05:51

Biology, 13.02.2020 05:51

Physics, 13.02.2020 05:51

Mathematics, 13.02.2020 05:51

Mathematics, 13.02.2020 05:51

Geography, 13.02.2020 05:51