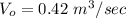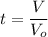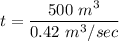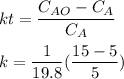A chemical breaks down in a flow-balanced, steady-state CFMR according to first-order reaction kinetics. At steady state, the upstream and downstream concentration of the chemical are 15 mg/L and 5 g/m3. Water is being treated at a rate of 0.42 m3/sec. The volume of the tank is 500,000 liters. Assuming a first-order reaction, what is the rate constant

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
A chemical breaks down in a flow-balanced, steady-state CFMR according to first-order reaction kinet...
Questions

Mathematics, 07.07.2019 12:30


Mathematics, 07.07.2019 12:30

Biology, 07.07.2019 12:30

Mathematics, 07.07.2019 12:30


Geography, 07.07.2019 12:30

Mathematics, 07.07.2019 12:30


Mathematics, 07.07.2019 12:30


Mathematics, 07.07.2019 12:30


History, 07.07.2019 12:30



Mathematics, 07.07.2019 12:30

Chemistry, 07.07.2019 12:30

Mathematics, 07.07.2019 12:30

Health, 07.07.2019 12:30

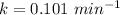
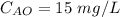
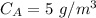 = 5 mg/L
= 5 mg/L