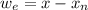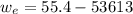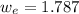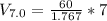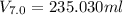A 72.0 g sample of an organic solid is dissolved in 180mL of water. The solid is extracted using one 60 mL extraction in the first extraction of an organic solvent which has a partition (distribution) coefficient with water of 10. The first extraction removed 55.4 g of solid from water. What are the numbers that need to go in box A and B to calculate the volume of solvent (y) that would be necessary to remove an additional 7.0g from the remaining sample dissolved in water. You DON'T have to complete the calculation to solve for y.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2

Chemistry, 23.06.2019 12:30
)a children’s liquid cold medicine has a density of 1.23 g/ml. if a child is to take 2.5 tsp in a dose, what is the mass in grams of this dose? (1 tsp = 5 ml)
Answers: 1
You know the right answer?
A 72.0 g sample of an organic solid is dissolved in 180mL of water. The solid is extracted using one...
Questions



History, 06.09.2019 04:20


History, 06.09.2019 04:20



Biology, 06.09.2019 04:20

English, 06.09.2019 04:20

English, 06.09.2019 04:20



English, 06.09.2019 04:20



Physics, 06.09.2019 04:20

English, 06.09.2019 04:20



Mathematics, 06.09.2019 04:20

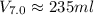
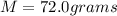
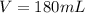
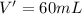
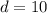
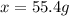
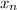 is mathematically given by
is mathematically given by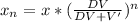
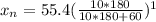
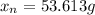
 is therefore
is therefore