
Chemistry, 19.04.2021 19:40 supasavb99
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to determine how many grams of aluminum oxide is formed during this reaction.
A.662.7 grams of Al2O3
B.24.6 grams of Al2O3
C.12.3 grams of Al2O3
D.6.1 grams of Al2O3
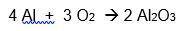

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to de...
Questions

Mathematics, 11.11.2020 23:40

Health, 11.11.2020 23:40

History, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40

Geography, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40

English, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40



Mathematics, 11.11.2020 23:40

Arts, 11.11.2020 23:40

Social Studies, 11.11.2020 23:40

History, 11.11.2020 23:40

English, 11.11.2020 23:40


English, 11.11.2020 23:40

English, 11.11.2020 23:40



