
Chemistry, 19.04.2021 20:40 carminamtzb3725
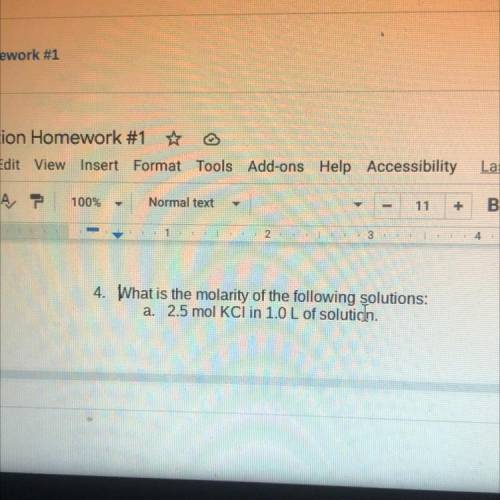
Does ANYONE get this? What is the molarity of the following solutions: a. 2.5 mol KCl in 1.0 L of solution,

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Does ANYONE get this? What is the molarity of the following solutions:
a. 2.5 mol KCl in 1.0 L of s...
Questions

History, 04.12.2020 02:40







Biology, 04.12.2020 02:40

Computers and Technology, 04.12.2020 02:40

Chemistry, 04.12.2020 02:40

History, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40


Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40


Mathematics, 04.12.2020 02:40

Mathematics, 04.12.2020 02:40



