Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
...

Chemistry, 19.04.2021 21:00 shyshy1791
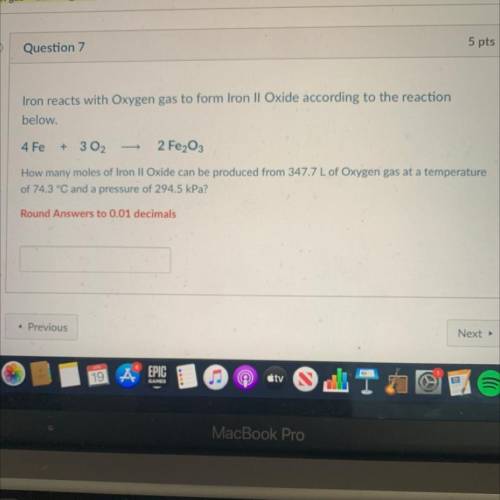
Iron reacts with Oxygen gas to form Iron II Oxide according to the reaction
below.
4 Fe
+ 3 02
2 Fe2O3
How many moles of Iron II Oxide can be produced from 347.7 L of Oxygen gas at a temperature
of 74.3 °C and a pressure of 294.5 kPa?
Round Answers to 0.01 decimals

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2
You know the right answer?
Questions

History, 17.12.2020 18:00

Biology, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00



Mathematics, 17.12.2020 18:00



Mathematics, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00





Mathematics, 17.12.2020 18:00


Mathematics, 17.12.2020 18:00


Mathematics, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00



