
Chemistry, 19.04.2021 21:20 skrillex88
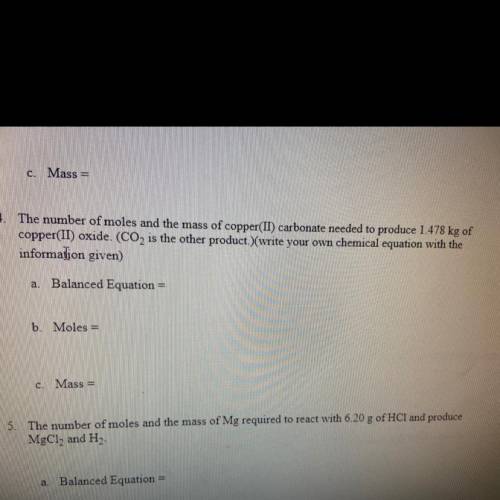
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two is the other products are your own chemical equation with the information given

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two...
Questions

Social Studies, 26.12.2019 08:31

English, 26.12.2019 08:31

Mathematics, 26.12.2019 08:31







Mathematics, 26.12.2019 08:31





Arts, 26.12.2019 08:31

History, 26.12.2019 08:31

Mathematics, 26.12.2019 08:31



Computers and Technology, 26.12.2019 08:31



